Learning Outcomes
i. Students will be able to explain the basic reactivity pattern of alcohols.
ii. Students will be able to identify the different types of reactions that alcohols can undergo.
iii. Students will be able to predict the products of alcohol reactions.
Introduction
Alcohols, organic compounds characterized by the hydroxyl (-OH) functional group, are versatile and reactive molecules that play a crucial role in various industries, including pharmaceuticals, solvents, and fuels. Understanding the reactivity of alcohols is essential for predicting their behavior in different chemical environments and utilizing their diverse synthetic applications.
i. Nucleophilic Substitution Reactions of Alcohols
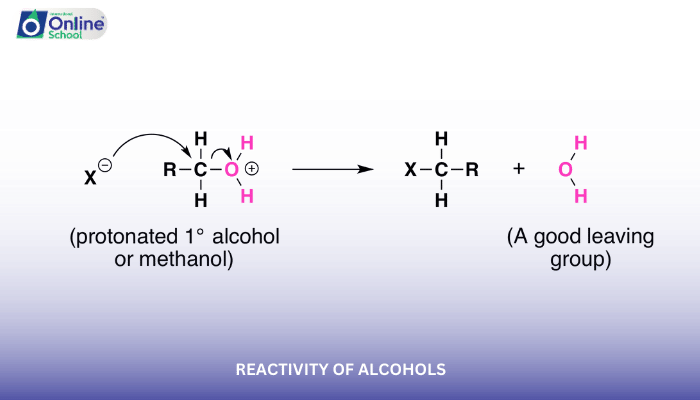
Alcohols can undergo nucleophilic substitution reactions, where the hydroxyl group (-OH) acts as a leaving group. These reactions typically involve the attack of a nucleophile (an electron-rich species) on the carbon atom attached to the hydroxyl group, resulting in the formation of a new functional group.
Dehydration of Alcohols: Alcohols can be dehydrated to form alkenes (compounds containing a carbon-carbon double bond) under acidic conditions. The type of alkene produced depends on the structure of the alcohol and the reaction conditions.
Substitution with Halides (Halogenation): Alcohols can react with halides (X2, where X = Cl, Br, I) in the presence of acids to form alkyl halides (compounds containing a halogen atom attached to an alkyl group). The reactivity of alcohols in this reaction depends on the order of the alcohol (primary, secondary, or tertiary).
Substitution with Sulfonyl Esters (Tosylation): Alcohols can react with p-toluenesulfonyl chloride (tosyl chloride, TsCl) in the presence of a base to form alkyl tosylates (compounds containing a tosylate group, -OTs). Tosylates are often used as intermediates in organic synthesis due to their good leaving group ability.
ii. Oxidation Reactions of Alcohols
Alcohols can undergo oxidation reactions, where the hydroxyl group (-OH) is converted to a carbonyl group (C=O). These reactions typically involve the use of oxidizing agents, such as potassium dichromate (K2Cr2O7) or sodium hypochlorite (NaClO), and result in the formation of aldehydes, ketones, or carboxylic acids.
Primary Alcohols to Aldehydes: Primary alcohols (alcohols with one alkyl group attached to the carbon atom bearing the hydroxyl group) can be oxidized to aldehydes.
Secondary Alcohols to Ketones: Secondary alcohols (alcohols with two alkyl groups attached to the carbon atom bearing the hydroxyl group) can be oxidized to ketones.
Tertiary Alcohols to Carboxylic Acids: Tertiary alcohols (alcohols with three alkyl groups attached to the carbon atom bearing the hydroxyl group) can be oxidized to carboxylic acids.
iii. Reduction Reactions of Alcohols
Alcohols can undergo reduction reactions, where the hydroxyl group (-OH) is converted to a methylene group (-CH2-). These reactions typically involve the use of reducing agents, such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4), and result in the formation of alkanes (compounds containing only single bonds between carbon atoms).
Aldehydes and Ketones to Alcohols: Aldehydes and ketones, carbonyl compounds containing a carbonyl group (C=O), can be reduced to alcohols using reducing agents. The type of alcohol product (primary or secondary) depends on the starting carbonyl compound.
The reactivity of alcohols is diverse and encompasses a range of reactions, including nucleophilic substitution, oxidation, and reduction. Understanding the factors that govern these reactions, such as the structure of the alcohol, the reaction conditions, and the choice of reagents, is essential for predicting the products and utilizing alcohols in various synthetic applications. From the production of pharmaceuticals to the development of new materials, alcohols continue to play a vital role in the field of organic chemistry.